Background
BKPyV is a pervasive human pathogen which resides within the reno-urinary tract in ~80-90% of the population. It is asymptomatic in healthy individuals; however, it poses a significant threat to immunosuppressed patients, especially those who have received a renal transplant or a hematopoietic stem cell transplant (HSCT).
According to the Global Observatory on Donation and Transplantation (ONT-WHO), an estimated 102,149 kidney transplants were performed worldwide in 2022. Furthermore, approximately 90,000 HSCTs are carried out globally each year, as reported by the World Wide Network of Blood and Marrow Transplantation (WHO-WBMT), and this number is on the rise. BKPyV reactivation is a common occurrence in these patients, resulting in serious complications like polyomavirus associated nephropathy (PVAN) and haemorrhagic cystitis (HC). Both conditions present a variable prevalence of up to 10%, posing a significant risk to transplant recipients. PVAN is linked with graft dysfunction and loss, whereas HC manifests as an inflammatory state of the urinary bladder, causing bleeding in the bladder wall. The lack of targeted treatments specifically designed for BKPyV presents challenges, leading to reliance on antivirals designed for other viruses, most of which prove ineffective or are toxic to renal cells. This, in turn, necessitates a reduction in the immunosuppressive drug regimen for patients, further increasing the risks of graft rejection.
To solve this problem, Prof. Colin Crump and his team from the Department of Pathology have demonstrated that inhibiting the activity of MAT2A, a critical human host gene for BKPyV replication, elicits a robust antiviral response. MAT2A inhibitors are currently in development for various cancer indications. In fact, companies such as Les Laboratoires Servier and IDEAYA Biosciences have already progressed to Phase II clinical trials for the treatment of malignancies like non-small cell lung cancer and colorectal cancer, providing safety data. Leveraging this existing knowledge, the focus now lies in establishing their effectiveness specifically against BKPyV. The groundbreaking discovery opens new avenues for antiviral drug development and offers a promising therapeutic option for BKPyV-associated diseases, addressing the limitations of available treatments.
Technology overview
- MAT2A is a Key Host Factor for BK Polyomavirus Infection:
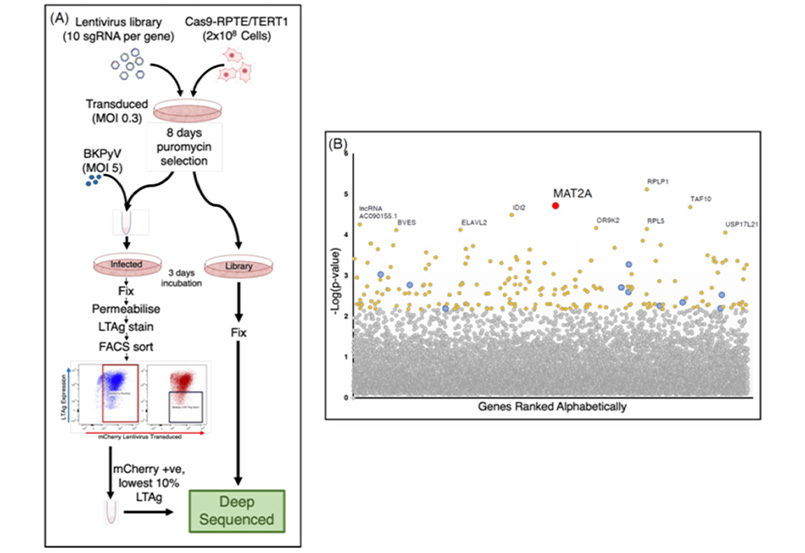
Figure 1: Experimental design of a CRISPR/Cas9 genome-wide screen, identifying MAT2A as a gene important for BKPyV early viral gene expression.
Using CRISPR-Cas9 technology, human renal epithelial cells (RPTE/TERT1) were screened to pinpoint host genes crucial for BKPyV infection (Figure 1A). After infection with BKPyV, cells were selected based on viral gene expression and compared to an uninfected reference library. Through next-generation sequencing, around 200 genes were found to be significantly associated with BKPyV infection. One of the most significant hits identified in the genome-wide screen was MAT2A (Figure 1B).
- Potent inhibition of BKPyV infection with a small molecule inhibitor of MAT2A:
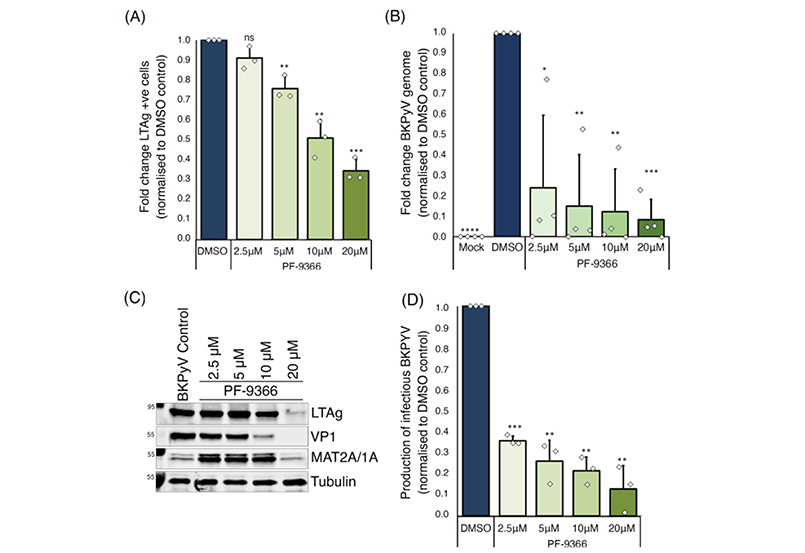
Figure 2: Inhibition of MAT2A with PF-9366 confirms importance of MAT2A activity for BKPyV replication.
To determine the effect of drug mediated MAT2A inhibition on BKPyV infection, RPTE/TERT1 cells were treated with various concentrations of the small molecule inhibitor PF-9366.
PF-9366 effectively reduced the number of cells expressing the key viral protein LTAg (Figure 2A) and decreased BKPyV genome copy numbers (Figure 2B) in a dose-dependent manner. Furthermore, MAT2A inhibition significantly impacted viral genome replication and late gene expression, with a >2.5-fold decrease in viral titres at the lowest dose (2.5μM) and a ~10-fold reduction at 20µM compared to control (Figures 2C and D). The observed reduction in viral titres aligns with diminished genome copy numbers, indicating that MAT2A inhibition primarily hinders BKPyV genome replication, resulting in reduced late gene expression and impaired virion assembly. Taken together, these results highlight the potential of MAT2A inhibition as an antiviral drug, effectively impeding BKPyV infection and offering a new therapeutic option for the treatment of BKPyV diseases.
Benefits
- Technology is the first specific treatment for BKPyV-associated diseases (e.g. PVAN and HC) in immunosuppressed patients, holding the potential to benefit around 20,000 affected transplant recipients annually.
- MAT2A inhibition holds promise in mitigating side effects (like nephrotoxicity and liver damage) associated with generic treatments, as it shows little effect on human renal epithelial cell metabolism or viability.
- MAT2A inhibitors, developed by a number of companies to treat tumour diseases, have progressed to Phase-II clinical trials, demonstrating safety in patients.
- MAT2A inhibition has potential efficacy against other BKPyV-related viruses such as JC polyomavirus (JCPyV) and Merkel cell polyomavirus (MCPyV). JCPyV, infecting 50-90% of the global population, often remains dormant, but in individuals with weakened immune systems, it can lead to progressive multifocal leukoencephalopathy (PML), a rare but fatal disease of the central nervous system affecting <1% of those with HIV/AIDS and < 0.1% on immunosuppressive drugs. MCPyV is believed to cause the majority of cases of the aggressive skin cancer Merkel cell carcinoma (MCC), with approximately 80% of MCC tumours found to be infected, though MCC itself is a rare disease affecting around 2,500 people annually in the US.
Opportunity
We are seeking a partner for this drug development or repurposing opportunity in the rare disease space. It is an attractive prospect since MAT2A inhibitors are currently in clinical development and have progressed to Phase-II clinical trials. We are seeking potential licensees and/or collaborators who can contribute to the successful development and commercialisation of MAT2A inhibition as a novel approach for the treatment of BKPyV-induced diseases. The next developmental stages involve building upon existing data to demonstrate the efficacy of this treatment in more advanced models (such as animal models or 3D organoids) and/or human samples (kidney tissue or organs). This will facilitate engagement in Phase-II clinical trials to demonstrate therapeutic efficacy.
References & Patents
Publication: Genome-wide CRISPR/Cas9 screen identifies MAT2A as a critical host factor for BK Polyomavirus (https://www.biorxiv.org/content/10.1101/2023.10.20.563362v1)
The use of MAT2A inhibitors and/or TSG101 inhibitors for treatment or prevention of viral infections has been covered by a patent (Patent number: PCT/GB2023/050875).