Cambridge Enterprise sister organisation CIC has made an investment in Gyroscope Therapeutics, an ophthalmology company developing genetically defined therapies for eye diseases. In February the company dosed its first patient in Phase I/II study, prompting media coverage.
A significant portion of Gyroscope’s founding intellectual property of came from the work of the laboratory of Professor Sir Peter Lachmann at the University of Cambridge. This IP was developed, licensed and invested in by Cambridge Enterprise.
As well as Professor Lachmann’s work, Gyroscope further leveraged the research of Dr David Kavanagh from Newcastle University and Professor Andrew Lotery at the University of Southampton to identify the patient population that would benefit from Gyroscope’s products.
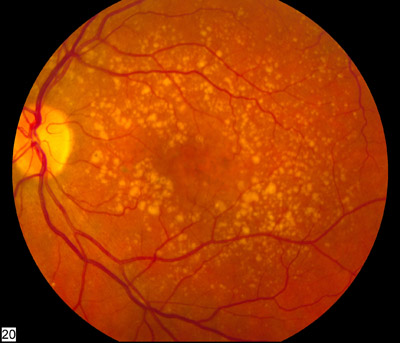
Gyroscope recently announced that the first patient had been successfully dosed in a Phase I/II clinical trial to assess the safety and biological activity of Gyroscope’s lead product, GT0005, which uses a novel therapeutic approach in dry age-related macular degeneration (AMD). This approach leverages knowledge of the genetic factors associated with advanced AMD and the biology of the inflammation that drives progression of the disease. Gyroscope’s gene therapy and surgical platform delivers an endogenous anti-inflammatory protein to the retina of patients. The goal is to develop a one-time treatment for the disease.
AMD is one of the leading causes of blindness. By the year 2020, an estimated 196 million people globally will have AMD and 11 million will have significant vision loss. Dry AMD, which is the most common form of AMD, is a slow deterioration of the cells of the macula which can lead to irreversible loss of visual function and eventual blindness. There are currently no approved treatments for dry AMD.
Dr Robert Tansley, Operating Partner at CIC, said, “The team at Gyroscope has developed an exciting and pioneering approach which aims to tackle a range of eye diseases with significant unmet medical need. While the clinical studies are very early stage and the treatment route is novel, the potential for these therapies, if successful, is enormous. As such, Gyroscope represents a great addition to the CIC portfolio and we look forward to supporting Soraya and her team as the business grows and develops.”
Dr Soraya Bekkali, Chief Executive Officer of Gyroscope Therapeutics, explained “Our goal at Gyroscope is to advance new therapies for the treatment of debilitating eye diseases such as age-related macular degeneration. Building on the research of Gyroscope’s scientific founders, we have been working relentlessly over the last two years to advance our first drug development programme into the clinic. We are delighted to have dosed the first patient in the FOCUS study. We believe this is a great step forward in developing a therapy to treat dry AMD, while we continue our efforts to expand our clinical programmes internationally.”