Background
In 2020, there were 1.9 million new cases of colorectal cancer, which is the 3rd most common cancer worldwide. Liver cancer and Leukaemia are the 6th and 13th most common cancer types, respectively. A game-changing drug targeting these cancers is yet to be discovered and the LGR5 monoclonal antibody is a promising candidate for the development of cutting-edge cancer treatments and immunotherapies.
Technology overview
LGR5 is a cell surface receptor that belongs to the GPCR family and whose transcript levels are low to undetectable in healthy tissues and elevated in several types of cancers. Recent studies suggest that increased expression of LGR5 may be one of the most common molecular alterations in cancers such as basal cell carcinomas, hepatocellular carcinomas, colorectal tumours, and ovarian tumours, amongst others. Targeting of cancer stem cells (CSCs) expressing LGR5 has therefore been highlighted as an attractive and versatile strategy for cancer treatment.
As a therapeutic target, LGR5 is relatively new but has been validated in a range of cancers with a therapeutic window of expression. The increased amounts of LGR5 expressed by CSCs makes LGR5 a marker of CSCs. Notably, CSCs are critical for the formation and maintenance of tumour growth and metastasis. This further strengthens the therapeutic merit of targeting LGR5 expressed by CSCs.
The LGR5 antibody-based strategies (ADC, BiTE and CAR-T) developed by Dr Marc de la Roche and Dr Maike de la Roche at the University of Cambridge hold promise as effective therapeutic treatment strategies for, in particular, colorectal cancer, hepatocellular carcinoma and acute lymphoblastic leukaemia.
Benefits
- The LGR5 antibody enables specific targeting of CSCs
- Demonstrates specificity to LGR5 over family members LGR4 and LGR6
- Demonstrates rapid internalisation and high affinity
- Utility as a platform for ADC, BiTE and CAR-T therapies in addition to being a valuable research tool
- Humanised antibody with in vivo proof of concept as an ADC
- Humanised antibody fragments (scFv-anti-LRG5) for BiTE and CAR-T constructs show extremely high binding affinity to its LGR5 epitope (Kd = 770 pM)
Applications
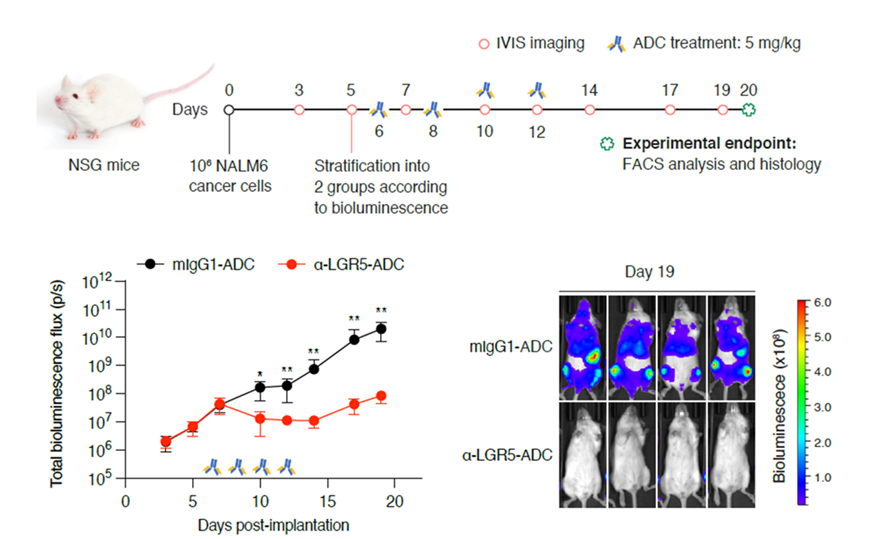
ADC in vivo studies
- Pre-B-ALL mouse model (NSG mice with NALM6 xenograft): treatment with anti-LGR5-ADC reduced tumour burden compared to control
- No tissue toxicity observed and proliferation of stem cells in the intestinal crypts was unaffected by anti-LGR5-ADC treatment
α-LGR5 Bispecific T cell Engagers (BiTEs)

- α-LGR5 BiTE activates T cells leading to efficient tumour cell destruction
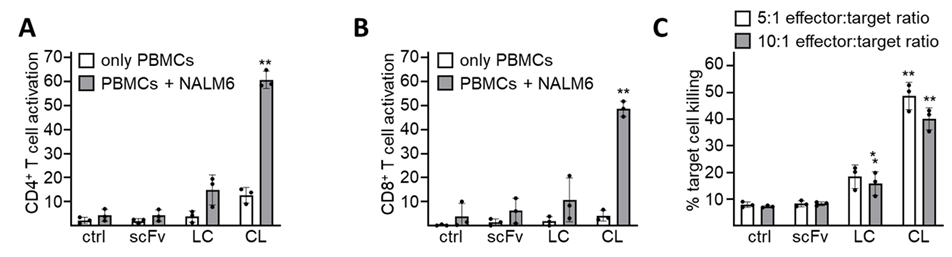
BiTE constructs were generated using the humanized a-LGR5 scFv and a humanized α-CD3 scFv in both directions (LC and CL, respectively).
A. CL- and LC-mediated activation of CD4+ T cells in healthy donor PBMCs in the presence or absence of NALM6 target cells determined as percent cells with combined expression of CD25 and CD69. Control (ctrl), no addition of molecule, scFv refers to treatment with the α-LGR5scFv Error bars represent SD for three independent CD4+ T cell activation assays. Significant differences in CD4+ activation in between addition of scFv and either the LC or CL BiTE molecules were determined by two-tailed t-test and shown at the **, p<0.001 level of significance.
B. CL- and LC-mediated activation of CD4+ T cells in healthy donor PBMCs in the presence or absence of NALM6 target cells determined as percent cells with combined expression of CD25 and CD69. Control (ctrl), no addition of molecule, scFv refers to treatment with the α-LGR5scFv fragment. Error bars represent SD for three independent CD4+ T cell activation assays. Significant differences in CD4+ activation in between addition of scFv and either the LC or CL BiTE molecules were determined by two-tailed t-test and shown at the **, p<0.001 level of significance.
C. CL- and LC-mediated activation of CD4+ T cells in healthy donor PBMCs in the presence or absence of NALM6 target cells determined as percent cells with combined expression of CD25 and CD69. Control (ctrl), no addition of molecule, scFv refers to treatment with the α-LGR5scFv fragment. Error bars represent SD for three independent CD4+ T cell activation assays. Significant differences in CD4+ activation in between addition of scFv and either the LC or CL BiTE molecules were determined by two-tailed t-test and shown at the **, p<0.001 level of significance.
α-LGR5-CAR NK cells
- Efficient killers with favourable killing kinetics
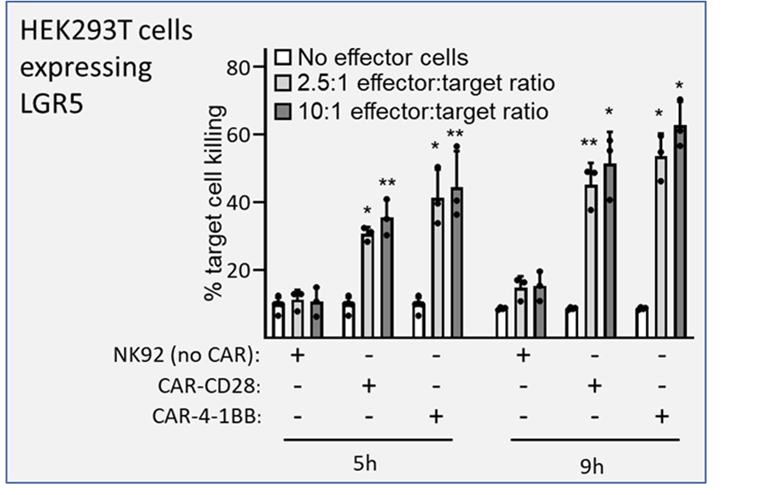
Cell killing activity of NK92 cells, or NK92 cells expressing α-LGR5-CAR-CD28- or α-LGR5-CAR-4-1BB. hLGR5-eGFP-overexpressing HEK293T target cells were incubated with effector NK92 cells at effector to target ratios of 2.5:1 and 10:1 for 5h or 9h, respectively. Error bars represent SD for three independent experiments. Significant differences in target cell killing were determined by two-tailed t-test and shown at *, p<0.05 and **, p<0.001. There were no significant differences in target cell killing between cellular ratios of 5:1 or 10:1.
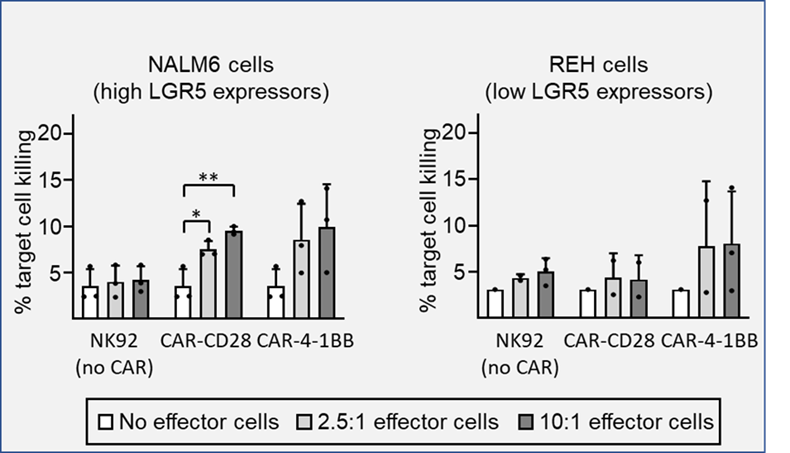
NALM6 and REH target cell killing activity of NK92 cells or NK92 cells expressing the α-LGR5-CAR-CD28 or the α-LGR5-CAR-4-1BB CARs with effector to target ratios of 2.5:1 and 10:1 after 12 hours incubation (top and bottom panels, respectively). Error bars represent SD for three independent experiments. Significant differences in target cell killing were determined by two-tailed t-test, at the *, p<0.05 and **, p<0.01.
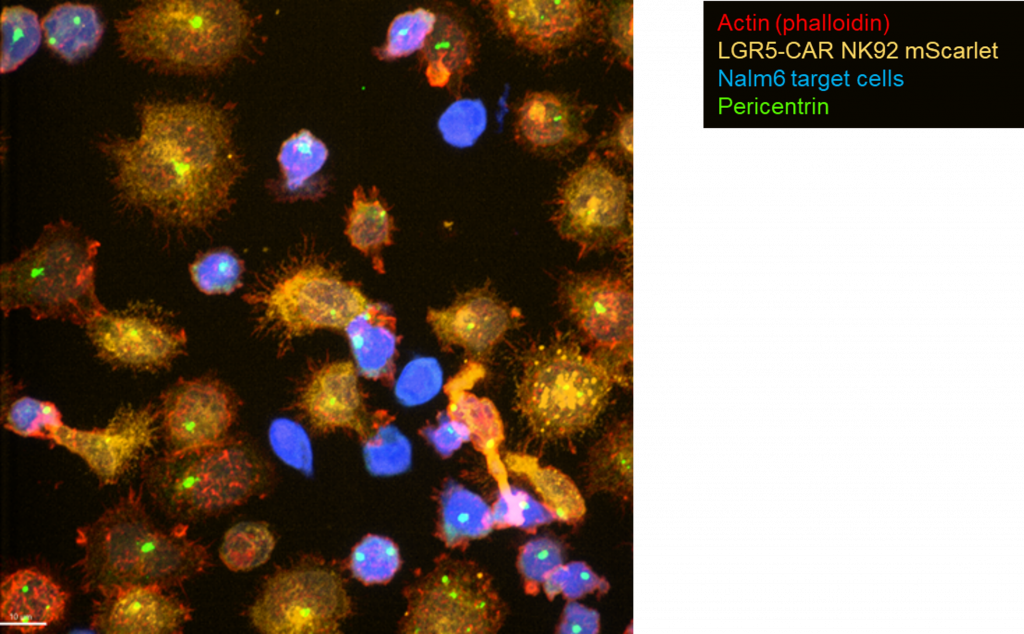
α-LGR5-CAR NK92 cells form productive synapses with tumour target cells
Opportunity
- Licensing and/or collaborative partnering opportunities available

Inventors
Maike de la Roche
Maike is a group leader at the CRUK Cambridge Institute. She completed her veterinary degree in Germany before pursuing her interest in immunology at the NIH and at the University of Cambridge. Her research group aims to understand the roles of the Hedgehog signalling pathway in immune cells, with the long-term goal of modulating these signals to improve immune responses in the context of cancer, infection, autoimmunity and vaccination.

Marc de la Roche
Marc is an Assistant Professor and Group Leader in the Department of Biochemistry, University of Cambridge. Prior, he was a Senior Research Scientist at the MRC Laboratory of Molecular Biology, Cambridge. UK.
Marc’s laboratory studies novel mechanisms regulating the activity of the Wnt signaling pathway and controlling biological roles in epithelial cell fate and morphology. The idea here is to identify molecular vulnerabilities in diseases associated with de-regulation of the Wnt signaling pathway that may lead to novel therapeutic strategies
Patents
Two patent applications filed:
- GB2202990.4
- GB2208544.3
Publications
For more information see:
LGR5 targeting molecules as therapeutic agents for multiple cancer types
Enquiry for LGR5 monoclonal antibody for cancer immunotherapy
Available Technologies Enquiry
To find out more about how this website collects your personal data see www.enterprise.cam.ac.uk/about-us/information-compliance/data-protection/core-privacy-notices/website-users-use-personal-information/

